Cell Cycle Indicator
Fluorescent Ubiquitination-based Cell Cycle Indicator (FUCCI)
FUCCI (Fluorescent Ubiquitination-based Cell Cycle Indicator) is a set of fluorescent probes which enables the visualization of cell cycle progression in living cells. FUCCI utilizes the phase-dependent nature of replication licensing factors Cdt1 and Geminin. A fusion protein of a fragment of Cdt1 (amino acids 30-120) with the fluorescent protein monomeric Kusabira-Orange 2 (mKO2) serves as an indicator of G1 phase. A fusion protein of a fragment of Geminin (amino acids 1-110 or 1-60) with the fluorescent protein monomeric Azami-Green 1 (mAG1) visualizes the S,G2 and M phase. In summary, FUCCI utilizes the highly selective, rapid degradation of the replication licensing factors mediated by the ubiquitin proteasome system to give excellent visualizations of the cell cycle.
Cdt1: Cdc10 dependent transcript 1 is a conserved replication factor required for licensing the chromosome for a single course of DNA synthesis. Abundantly expressed throughout the cell cycle, Cdt1 is ubiquitinated by the ubiqutin ligase complex SCFskp2 during S and G2 phases and degraded by proteasome.
Geminin: Geminin inhibits the licensing activity of Cdt1. Geminin interferes with the binding of licensing factors to the replication origin once a chromosome has started to replicate during the S phase. During M and G1 phases, Geminin is ubiquitinated by the E3 ligase complex APCcdh1 and degraded by the proteasome.
What makes FUCCI a powerful research tool?
- Real-time visualization of cell cycle progression
- Spatio-temporal imaging of cell cycle dynamics
- Utilization of florescent proteins Green mAG1and Orange mKO2
- Fucci-G1 Orange labels G1 phase nuclei in orange. Fucci-S/G2/M Green labels S/G2/M phases nuclei in green
By visualizing the cell cycle, Fucci is a powerful tool to investigate any process that has to do with cell growth and differentiation, such as the development and regeneration of organs as well as carcinogenesis.
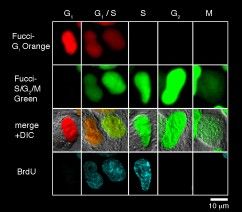
Each cell cycle of the G1, G1/S , S, G2, and M phases can be determined by the combination of Fucci-G1 Orange, Fucci-S/G2/M Green, and an antibody against PCNA (Proliferation Cell Nuclear Antigen). G1 phase is indecated by orange. Both orange and green were observed in the G1/S phase. Additional immunostaining color by PCNA was observed at the initiation of the S phase. Cells with pure green fluorescence were either in the S or G2 phase and were distinguished by immunostaining of the S phase. The rest of the cells were classified into the M phase. These results are consistent with the fact that Cdt1 accumulates in G1, while Geminin accumulates in S/G2/M. (Provided by Dr. Sakaue-Sawano at RIKEN. Cell (2008) 132:487-98).
Applications
High Content Imaging
FUCCI can be used for the visualization and analysis of single cell metrics which can provide rich data for analysis.
Cancer Inhibitor Screening
FUCCI was used to test the effect of selinexor on cancer cell growth. After imaging the cell cycle effects of selinexor using FUCCI, it was determined that G-1 or early S-phase treated cells show the strongest response and most often die or arrest.
Publications
Madrigal, Pedro, et al. “Epigenetic and transcriptional regulations prime cell fate before division during human pluripotent stem cell differentiation.” Nature Communications 14.1 (2023): 405.
Zhou, Xiaokun, et al. “Downregulation of CDC25C in NPCs Disturbed Cortical Neurogenesis.” International Journal of Molecular Sciences 24.2 (2023): 1505.
Liu, Zhiqing, et al. “Hedyotis diffusae Herba-Andrographis Herba inhibits the cellular proliferation of nasopharyngeal carcinoma and triggers DNA damage through activation of p53 and p21.” Cancer Gene Therapy 29.7 (2022): 973-983.
Hoffman, Robert M., and Shuya Yano. “Tumor-specific S/G 2-phase cell cycle arrest of cancer cells by methionine restriction.” Methionine Dependence of Cancer and Aging: Methods and Protocols (2019): 49-60.
Hidayat, Moulid, et al. “Role of FBXW7 in the quiescence of gefitinib-resistant lung cancer stem cells in EGFR-mutant non-small cell lung cancer.” Biomolecules and Biomedicine 19.4 (2019): 355-367.
Yano S, Hoffman RM. Real-Time Determination of the Cell-Cycle Position of Individual Cells within Live Tumors Using FUCCI Cell-Cycle Imaging. Cells. 2018 Oct 14; 7(10). PMID: 30322204.
Hoffman RM, Yano S. In Vivo-Like Cell-Cycle Phase Distribution of Cancer Cells in Gelfoam® Histoculture Observed in Real Time by FUCCI Imaging. Methods Mol Biol. 2018; 1760:109-123. PMID: 29572799.
Yano S, Takehara K, Tazawa H, Kishimoto H, Urata Y, Kagawa S, Fujiwara T, Hoffman RM. Therapeutic Cell-Cycle-Decoy Efficacy of a Telomerase-Dependent Adenovirus in an Orthotopic Model of Chemotherapy-Resistant Human Stomach Carcinomatosis Peritonitis Visualized With FUCCI Imaging. J Cell Biochem. 2017 11; 118(11):3635-3642. PMID: 27171483.
Yano S, Takehara K, Tazawa H, Kishimoto H, Urata Y, Kagawa S, Fujiwara T, Hoffman RM. Efficacy of a Cell-Cycle Decoying Killer Adenovirus on 3-D Gelfoam®-Histoculture and Tumor-Sphere Models of Chemo-Resistant Stomach Carcinomatosis Visualized by FUCCI Imaging. PLoS One. 2016; 11(9):e0162991. PMID: 27673332.
Yano S, Takehara K, Tazawa H, Kishimoto H, Urata Y, Kagawa S, Fujiwara T, Hoffman RM. Cell-cycle-dependent drug-resistant quiescent cancer cells induce tumor angiogenesis after chemotherapy as visualized by real-time FUCCI imaging. Cell Cycle. 2017 Mar 04; 16(5):406-414. PMID: 27715464.