p62 ELISA Kits and Antibodies
p62/SQSTM1 is thought to be a substrate that binds directly to LC3 causing its selective degradation by autophagy. An anti-p62/SQSTM1 antibody, which has a variety of applications including Western blotting (WB), Immunoprecipitation (IP), Immunocytochemistry (ICC), and Immunohistochemistry (IHC), is useful for observation of p62-caused pathogenesis and dynamics at the molecular level.
The ubiquitin-conjugating protein p62/SQSTM1 is thought to be a scaffold protein because it interacts with variety of molecules involved in toll-like receptor (TLR) signaling, such as TRAF6, ERK, and aPKC. Recent evidence shows that p62 binds directly to the autophagosome marker LC3 and is then selectively degraded by autophagy. In liver- or brain-specific autophagy-defective mice, excessive p62 accumulation leads to formation of ubiquitin- and p62-positive inclusions. Importantly, ubiquitin- and p62-positive inclusions have been detected in tissues from patients with neurodegenerative diseases (such as Alzheimer’s disease, Parkinson’s disease, and amyotrophic lateral sclerosis), alcoholic hepatitis, fatty liver, or liver cancer.
Currently, the association of autophagy-induced p62 metabolic failure with the pathogenesis of these diseases is an important focus of study.
Anti-p62 Antibodies:
Highly cited, for multiple applications: Code No. PM045 (Polyclonal), M162-3 (Monoclonal)
Minimal background signal in western blotting: Code No. PM066 (Polyclonal)
For Immunofluorescence (IF): Code No. M162-A48, M162-A59 & M162-A64 (Monoclonal)
New p62 ELISA Kit:
Total p62: Code No. CY-7055
Phosphorylated p62
Hot topic in research on neurodegenerative disease and cancer
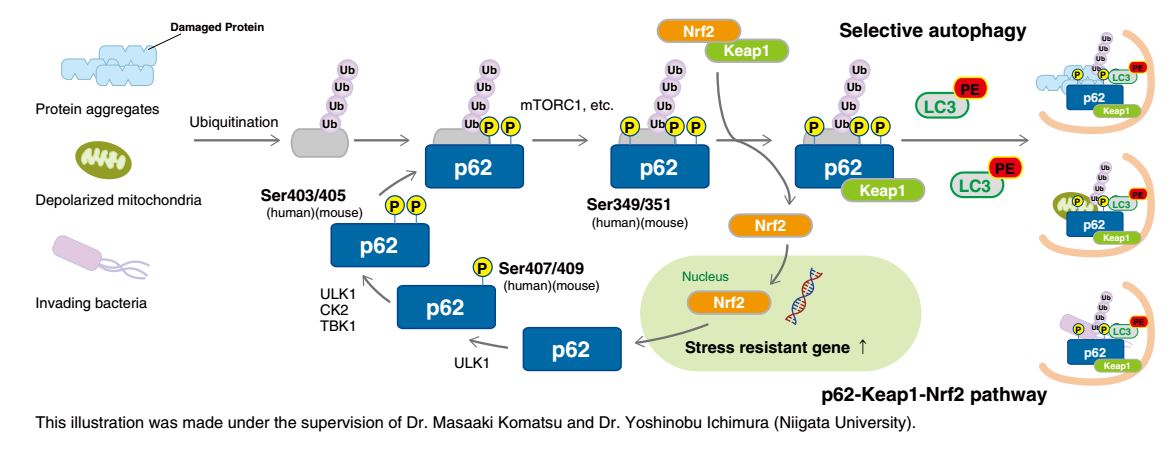
p62 contains multiple phosphorylation sites. Sequential phosphorylation of these sites regulates biological defense mechanisms such as selective autophagy.
The phosphorylation of Ser407 (human)/Ser409 (mouse) precedes the phosphorylation of Ser403 (human)/Ser405 (mouse) in p62, which increases its affinity for poly ubiquitin chains. Consequently, ubiquitinated abnormal protein aggregates, depolarized mitochondria, and invading intracellular bacteria are sequestered by phospho-p62. Further phosphorylation of Ser349 (human)/Ser351 (mouse) by mTORC1 increases the affinity of p62 for Keap1, inducing dissociation of Nrf2 from Keap1 and nuclear translocation of Nrf2 (the p62-Keap1-Nrf2 pathway). Nrf2 is a stress-response transcription factor and activates the transcription of various stress resistance genes. Nrf2 also induces p62 gene expression, forming a positive feedback loop. Phospho-p62 with bound Keap1 interacts with LC3 through the LIR (LC3-interacting region) and is degraded by the autophagy pathway. Thus, the cells under stress conditions effectively overcome their negative environment by activating two biological defense mechanisms through the phosphorylation of p62.
Impaired selective autophagy is implicated in various diseases. For example, neurons in familial parkinsonism fail to clear protein aggregates and depolarized mitochondria, resulting in neuronal damage and compromised brain function. In hepatocarcinoma cells, p62 is constitutively phosphorylated at Ser349, causing continuous activation of Nrf2. Hence, inhibitors of p62 phosphorylation and inhibitors of the interaction between phospho-p62 and Keap1 have the potential to be novel cancer therapeutics. (Reference: Saito, T., et al., Nat. Commun. 7, 12030 (2016) PMID: 27345495).
Anti-Phospho-p62 Antibodies:
Ser403: Code No. D343-3 and D344-3 (Monoclonal)
Ser351: Code No. PM074 (Polyclonal)
New Anti-Phospho-p62 ELISA Kits:
Ser349: Code No. CY-7056
Ser403: Code No. CY-7057
Related Products
Anti-NRF2 Antibodies: Code No. PM069 (Polyclonal) and M200-3 (Monoclonal)
Anti-KEAP1: Code No. M224-3 (Monoclonal)





