Structure of antibodies
 The variable region and the constant region
The variable region and the constant region
The N-terminal domains of the H and L chains are called the variable regions (V regions), and the rest of the molecule is called the constant region (C region). The amino acid sequence of the V region varies from antibody to antibody, accounting for the high degree of three-dimensional structural diversity of immunoglobulin chains.
Because of this diversity, antibodies bind to a wide range of antigens. (The diversity of antibodies is described later.)
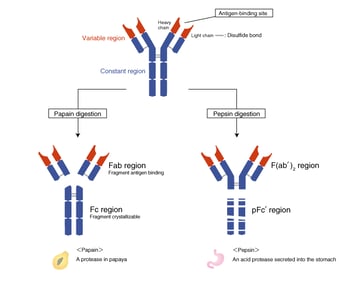
The Fab region and the Fc region
The protease papain cleaves antibodies disulfide bonds that connect the two H chains (the hinge region), generating three fragments. The two N-terminal fragments are called the Fab region, and the C-terminal fragment is called the Fc region. The “ab” in Fab stands for “antigen binding.” The “c” in Fc stands for “crystallizable,” because the well-conserved amino acid sequence allows this fragment to crystallize.
The F(ab’)2 region and the pFc’ region
Another protease pepsin cleaves antibodies and generates an N-terminal fragment with the hinge region still attached. This fragment is called the F(ab’)2 (fab two prime) region. The antigen-binding site is called Fab’ (fab prime) to distinguish it from the Fab region that does not include the hinge region. The pepsin fragment is designated F(ab’)2 because the two Fab’ regions remain connected at the hinge region. The C-terminal pFc’ region is digested into small fragments by pepsin.
Related Links
*Antibody
→Antibody basics
→Antibodies as a research tool
- How to select antibodies
- Labeled antibodies
- How to label antibodies
- Main causes of non-specific reactions
- How to reduce non-specific reactions
- Tags and Tag antibodies
*Qualitative and quantitative measurements of proteins using antibodies
- Western blotting (WB)
- Enzyme-linked Immunosorbent assay (ELISA)
- Immunoprecipitation (IP)
- Co-immunoprecipitation (Co-IP)